
doimasao[at]pharm.kyoto-u.ac.jp
2018- Professor, Kyoto University
2011- Associate Professor, Kyoto University
2007- Lecturer, Kyoto University
2006- Assistant Professor, Kobe University
2004- Research Fellow, IGBMC, CNRS, France
2003- Ph.D., The University of Tokyo
Publication list
Associate Editor, Biological Timing and Sleep, npj series
Research Projects

My main research focus is on understanding the ways in which deregulation of the body clock (‘circadian clock’) drives development of human diseases. This is an extremely pressing matter, given the stresses that modern lifestyles exert on the circadian system, such as increased use of artificial light at night and the impact of shift work and ageing. As such, my laboratory is committed to developing pharmacological strategies that aim to realign or rejuvenate intrinsic timekeepers, and thereby act as therapeutic interventions against conditions associated with circadian misalignment such as cancer, obesity, and heart disease. Keep exploring unexpected seeds for unmet medical needs!
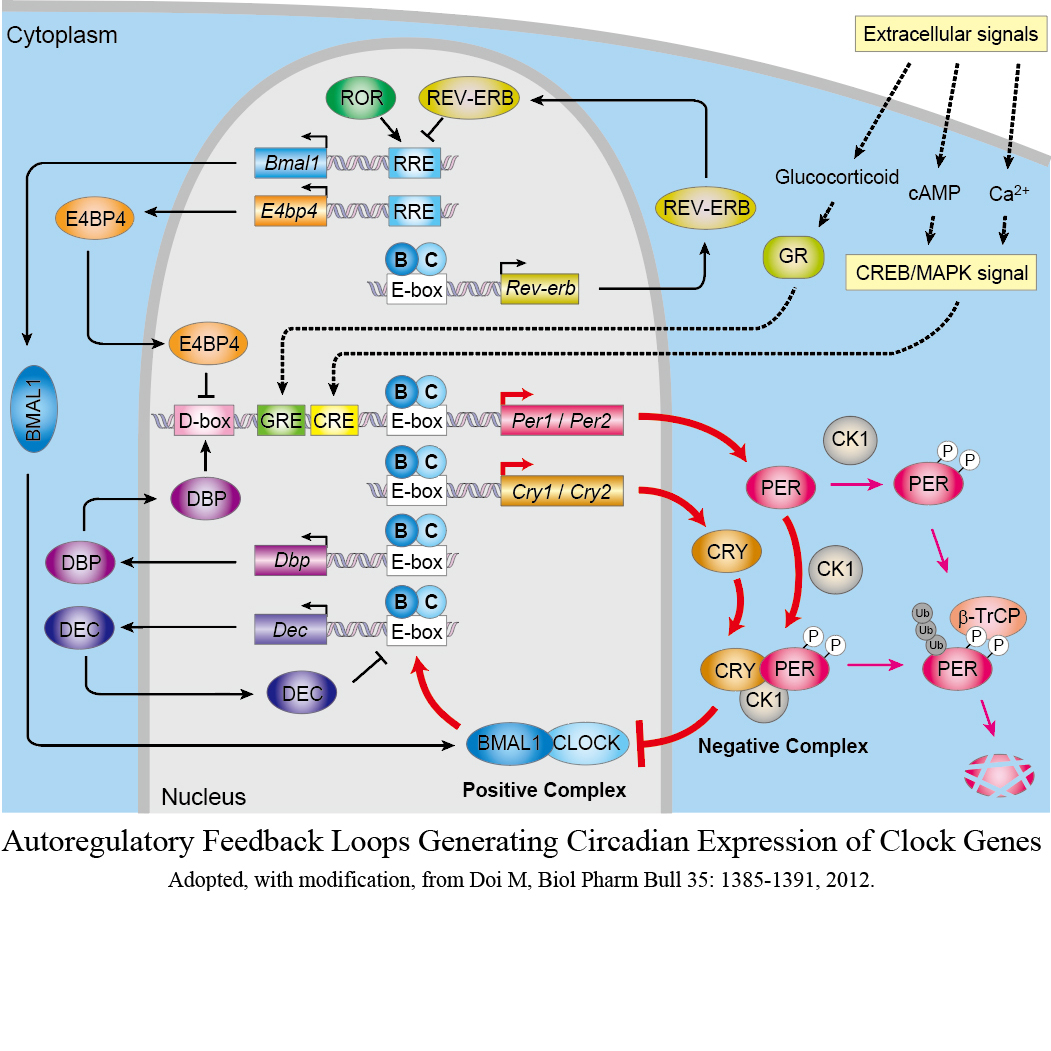
State-of-the-art research on circadian rhythms began with a seminal finding of “clock genes” that are common to human beings and other mammals. The field since then has expanded into a rich and diverse interdisciplinary academic field that deals with both basic sciences and clinical interrogations of daily rhythms in physiology. There are still a number of mysteries in the molecular clock gears, which would reveal as yet undeciphered molecular links between daily physiologies and circadian clock.
See: Doi et al PNAS, 2001; Doi et al Curr Biol, 2004; Doi et al Cell, 2006; Doi et al Nature Neurosci, 2006; Fustin et al Cell, 2013

A section of the hypothalamus called the suprachiasmatic nucleus (SCN) lies at the center of the body’s master clock and gets input directly from light sensors in the eyes, keeping the rest of the body on schedule. My lab studies the molecules, cells, and circuits underlying these circadian rhythms in the SCN using techniques that include real-time cellular imaging and multiple genetic manipulations, i.e. mutants, knockouts, transgenics, optogenetics, etc. This approach is giving new insight into the roles of specific neuropeptides and G-protein-coupled receptor-mediated signaling circuits in the rich repertoire of daily behaviors and physiologies.
See: Doi et al Nature Commun, 2011; Yamaguchi et al Science, 2013
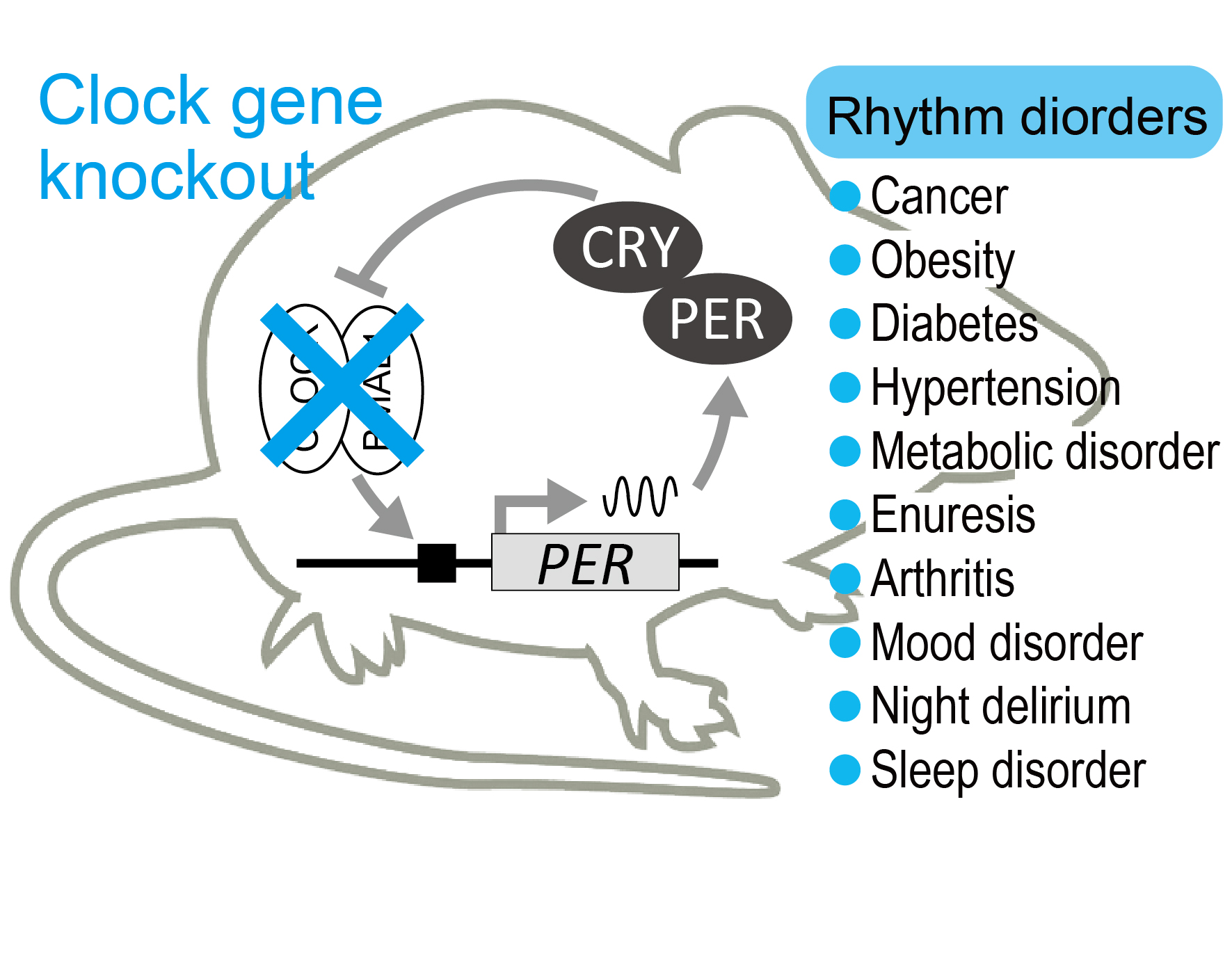
One of the most significant conceptual changes brought about by the analysis of circadian clock-deficient mice is that abnormalities in the circadian clock are linked not only to sleep arousal disorder but also to a wide variety of common diseases, including hypertension, diabetes, obesity, and cancer. Our lab previously revealed a molecular mechanism linking circadian malfunction to salt-sensitive hypertension. Elucidation of clock dysfunction behind diseases will continue to be a main project of our team.
See: Doi et al Nature Med, 2010; Negoro et al Nature Commun, 2012; Chao et al Nature Commun, 2017

What will be a representative medical application of time control medicine? Sleep-wake cycles are profoundly influenced by the abnormalities of the circadian clock. We have recently demonstrated that Gpr176 is an orphan G-protein-coupled receptor that sets the pace of the central clock in the brain. Gpr176 is thus a potential drug target to treat sleep-wake cycle dysfunction. Development of a chemical modulator of Gpr176 function is our next on-going challenge.
See: Doi et al Nature Commun, 2016; Wang et al Sci Rep, 2020; Nakagawa et al IJMS, 2020

Another unique feature of the circadian clock is that it could provide non-pharmacologic treatment options: without developing drug, personal body clock profiles can be used to enhance health and even reduce drug consumption. Potential applications would include increment of productivity of working and/or learning based on individual chronotypes such as propensity for morning versus evening and optimal duration of sleep. In an aging society, it will be also important to develop “successful aging” protocol by taking advantage of the circadian clock as a low-cost health maintenance time-keeper. It's time to take time seriously.
See: Sasaki et al Nature Aging, 2022
We identified a DNA switch required for daily regulation of behavior and physiology. To do so, we generated mutant mice carrying a mutation only at the E′-box cis-element in the promoter region of the core clock gene Per2. Although Per2 proteins remain intact, this non-coding E′-box mutation abolishes molecular clock oscillation and renders circadian locomotor activity and body temperature rhythm unstable. The discovery of the non-coding DNA switch required for circadian rhythm may provide clues to elucidate the genetic variance or polymorphism between morning person and evening person in humans. See: Doi & Shimatani et al Nature Commun, 2019
The development of anti-cancer drugs and therapies for lifestyle-related illnesses, such as cardiovascular disorder, obesity, and diabetes mellitus, would not solely involve manipulation of the core clock proteins per se, but will also be equally facilitated through the manipulation of the function of the group called "output" genes, which are regulated by the circadian clock. Manipulating the "input" signals that adjust the phase and amplitude of the circadian clock will also expand the path to drug discovery for clock-related disorders.
See: Doi et al J Clin Endocrinol Metab, 2014; Ota et al Mol Cell Biol, 2014

The body temperature rhythm is one of the most conspicuous outputs of the circadian clock and is crucial for maintaining homeostasis in metabolism and sleep. We showed that the calcitonin receptor (Calcr) mediates body temperature rhythm during the active phase in mice. Importantly, a specific subset of neurons in the center of the circadian clock express the Calcr.
See: Goda & Doi et al Genes Dev, 2018

Do you usually wake up early or sleep in the morning? Genome-wide association studies featured RGS16 as a “morning person”-associated gene. These human studies corroborate our original research finding using knockout mice. RGS16 shows strong circadian expression in the brain's pacemaker neurons and its deletion leads to elongation of period of circadian locomotor activity rhythm. We are currently interested in defining brain G-protein signaling that sets the time to go to sleep and wake up every day.
See: Doi et al Nature Commun, 2011
cf. Jones et al Nature Commun, 2019

Applications of the circadian clock are not limited to the development of new drugs. We are also interested in a "circadian medicine" approach to tailor existing therapies according to the internal body clocks for better clinical outcomes. Symptoms of illness and the efficacies of drugs are known to change according to time of the clock in our body and can therefore be predicted. It is hoped that the body time information will be used as clinical evidence to maximize the effectiveness of existing drugs and also to reduce their potential adverse side-effects.


